Fact.MR, in its latest market study, opines that the Sales of home diagnostics will grow at a healthy 3.5% value CAGR, surpassing US$ 6.3 million by 2026.
Companies in the Home Diagnostics Market are facing issues in keeping their production facilities fully functional due to shortage of staff and resources amidst the COVID-19 (Coronavirus) outbreak. Get a hands-on over key drivers and threats to the Home Diagnostics Market to make your company future-ready post the pandemic. Avails out reports for exciting prices to learn new opportunities that companies can capitalize on during and after the Coronavirus crisis.
For detailed insights on enhancing your product footprint, request a sample here – https://www.factmr.com/connectus/sample?flag=S&rep_id=1892
Fact.MR, in its latest market study, opines that the home diagnostics market will grow at a healthy 3.5% value CAGR, surpassing US$ 6.3 million by 2026. According to the report, sales will remain buoyant in developed regions, with North America and Europe collectively accounting for over 50% revenue share of the market. The Fact.MR study remains bullish on the prospects of home diagnostics market players in developing countries, as growing focus on health & wellness, and emergence of many Asian countries as medical tourism hubs creates opportunities.
Increasing preference for self-diagnosis continues to be a trigger for home diagnostics sales, whereas a combination of industry-specific and regulatory factors continue to influence the strategies of market players. Governing bodies such as the U.S. Food and Drug Administration (FDA) and the European Commission regulate home diagnostics with stringent quality control regulations and labeling requirements.
For critical insights on this market, request for methodology here – https://www.factmr.com/connectus/sample?flag=RM&rep_id=1892
Market Segmentation:
By Test Type
- Glucose Monitoring Devices
- Pregnancy Test
- HIV Test Kits
- Ovulation Predictor Test Kits
- Cholesterol Detection Test Kits
- Drug of Abuse Test Kits
By Form
- Cassettes
- Strips
- Midstream
- Digital Monitoring
- Instruments
- Test
- Dip Cards
By Regional Market and Country-wise Segmentation
- North America
- USA
- Latin America
- Europe
- France
- Asia Pacific
- Middle East
- Africa
- China
By Distribution Channel
- Retail Pharmacies
- Drug Stores
- Hypermarkets / Supermarkets
- Online Pharmacies
For instance, Abbott Laboratories, a leading pharmaceutical company in the U.S., recently got an FDA approval for marketing its FreeStyle® Libre 14 day Flash Glucose Monitoring system in the U.S. ACON Laboratories, another prominent player in the home diagnostics market announced that it has received FDA clearance of Mission® U120 urine reagent strips. According to the report, compliance to ever-evolving regulations and gaining regulatory approval remains both an opportunity and challenge for market players.
The Fact.MR study finds that glucose monitoring devices will continue to witness robust demand and dominate the home diagnostics market with over than 80% revenue share in the market. According to the World Health Organization (WHO), the number of people suffering from diabetes reached 422 million in 2014, and it will soon become a leading cause of death. Increasing prevalence of diabetes worldwide and the growing need for monitoring sugar levels among diabetes patients is likely to boost adoption of glucose monitoring devices during the assessment period. Home diagnostics market players are aware of the surging demand for glucose monitoring devices, and are focusing on increasing their product portfolio in this segment. The other lucrative segments in the home diagnostics market include pregnancy tests and ovulation prediction kits.
For comprehensive insights on this market adoption, ask an analyst here – https://www.factmr.com/connectus/sample?flag=AE&rep_id=1892
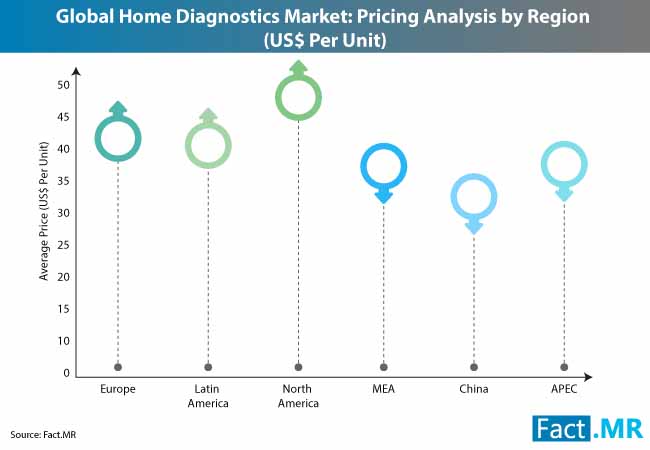
According to the report, although demand for home diagnostics is concentrated in developed countries, home diagnostics market players are putting efforts to boost uptake in developing regions. According to statistics published by WHO, the prevalence of diabetes has been the highest in middle and low-income countries.
Anticipating the burgeoning demand for glucose monitoring devices in emerging Asia, players in the home diagnostics market are shifting their focus towards developing nations, including China and India. ARKRAY, Inc. – a Japanese manufacturer in the home diagnostics market – recently announced that it will strengthen its diabetes testing instrument production capacity in China. Leading manufacturers in the home diagnostics market are modifying their marketing strategies to improve awareness about cost-effective and efficient home diagnostics to leverage lucrative opportunities in the Asia-Pacific region.
Read More Trending and Similar Reports from Fact.MR – https://www.globenewswire.com/en/news-release/2019/09/11/1914197/0/en/Peak-Flow-Meters-to-Ride-on-Portability-Quotient-Gains-Remain-Driven-by-Introduction-of-Smart-and-Efficient-Variants-Fact-MR.html
About Us:
Market research and consulting agency with a difference! That’s why 80% of Fortune 1,000 companies trust us for making their most critical decisions. While our experienced consultants employ the latest technologies to extract hard-to-find insights, we believe our USP is the trust clients have on our expertise. Spanning a wide range – from automotive & industry 4.0 to healthcare & retail, our coverage is expansive, but we ensure even the most niche categories are analyzed. Our sales offices in United States and Dublin, Ireland. Headquarter based in Dubai, UAE. Reach out to us with your goals, and we’ll be an able research partner.
Contact:
US Sales Office:
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
Corporate Headquarter:
Unit No: AU-01-H Gold Tower (AU),
Plot No: JLT-PH1-I3A,
Jumeirah Lakes Towers,
Dubai, United Arab Emirates
Email: sales@factmr.com
Visit Our Website: https://www.factmr.com